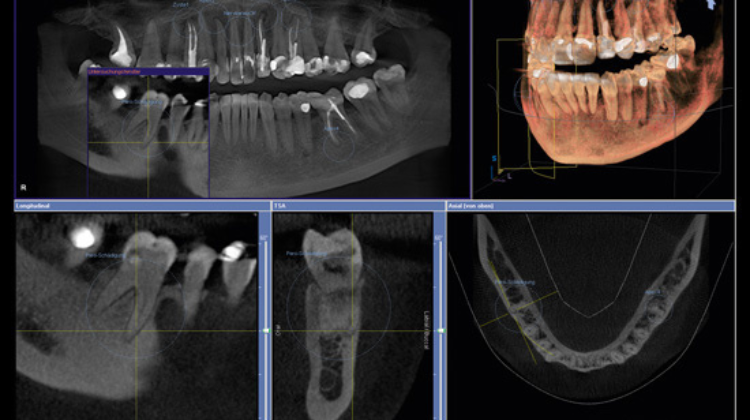
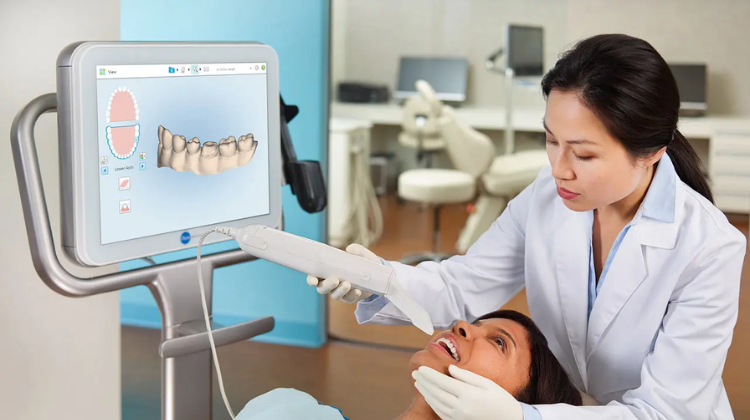
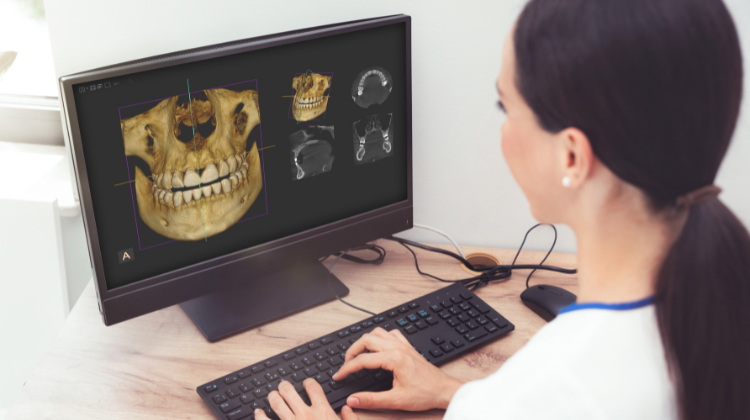
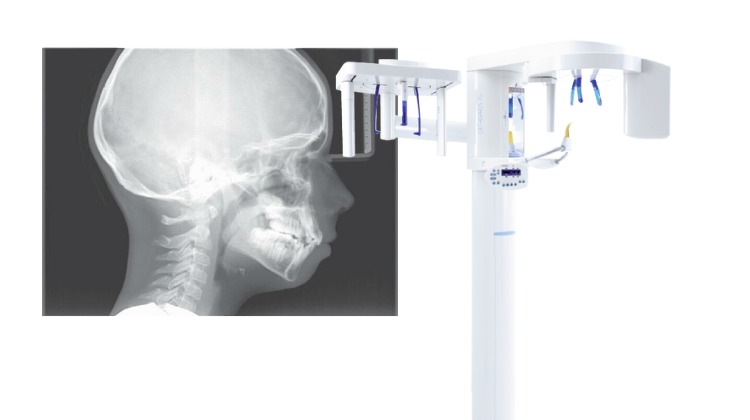
Maximizing Your Investment: Upgrading to Dental CBCT
Upgrading to CBCT is not just a technological upgrade—it's an investment in precision, efficiency, and patient-centric care. By harnessing the power of CBCT imaging, dental practices can elevate diagnostic accuracy, treatment planning efficacy, and patient satisfaction.